Frontiers, Regulation of lipid signaling by diacylglyceride ( DAG) kinases during T-cell development and function. Sruti Krishna et al. 2015
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0102526
Visualization of phosphatidic acid (PA) fluctuation in plasma membrane of a living cells. July 15, 2014
Abstract
We developed genetically-encoded fluorescent sensors based on Förster Resonance Energy Transfer to monitor phosphatidic acid (PA) fluctuations in the plasma membrane using Spo20 as PA-binding motif. Basal PA levels and phospholipase D activity varied in different cell types. In addition, stimuli that activate PA phosphatases, leading to lower PA levels, increased lamellipodia and filopodia formation. Lower PA levels were observed in the leading edge than in the trailing edge of migrating HeLa cells. In MSC80 and OLN93 cells, which are stable cell lines derived from Schwann cells and oligodendrocytes, respectively, a higher ratio of diacylglycerol (DAG) to PA levels was demonstrated in the membrane processes involved in myelination, compared to the cell body. We propose that the PA sensors reported here are valuable tools to unveil the role of PA in a variety of intracellular signaling pathways.Introduction
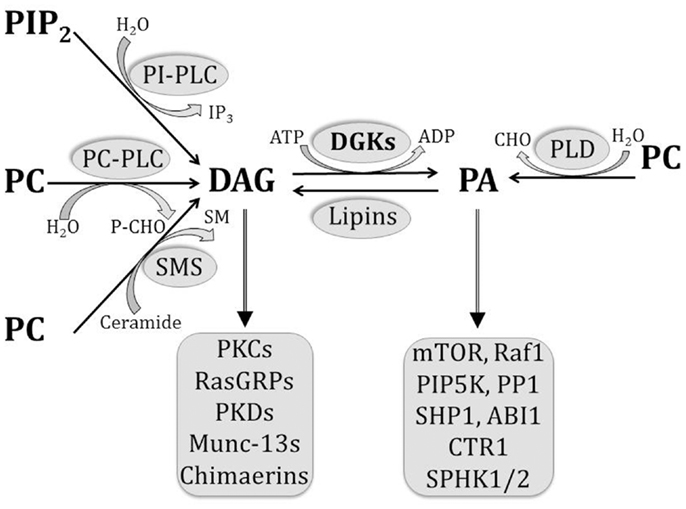
Phosphatidic acid (PA) is an acidic phospholipid that plays a central role in the biosynthesis of other lipids. By serving as a substrate or by modulating the activity of various enzymes, it participates in the complex network of structural, energy storage, and signaling lipids [1]. Using phosphatidylcholine as a substrate, PA can be synthesized by phospholipase D (PLD), and converted into diacylglycerol (DAG) by PA phosphatases. DAG can be converted back into PA by DAG kinases (DGK). Moreover, PA can be metabolized by phospholipase A2 to generate lysophosphatidic acid (LPA), whereas the reverse reaction is catalyzed by lysophosphatidic acid acyl transferases [1]–[3] (see Fig. S1). In addition, PA itself is a lipid mediator [3], and its growing list of effector molecules includes proteins involved in cytoskeleton rearrangement, vesicle trafficking, cell growth, spreading, proliferation, and survival [2], [3]. Importantly, with the exception of PLDs, the above mentioned enzymes either render or metabolize another signaling lipid, thus exerting a signaling-switch activity between PA and other pathways. Moreover, PA is a small cone-shaped phospholipid that provides flexibility to cellular membranes. It stabilizes the negative curvature of lipid bilayers, helping in the formation of vesicles from Golgi apparatus or plasma membrane [4], and mediating fusion and fission events of organelles such as mitochondria [5].
Traditionally, PA levels have been measured using thin-layer chromatography or liquid chromatography coupled to mass spectrometry [6], [7]. However, these techniques do not provide the desired spatio-temporal resolution for some applications. Further, variations in the signaling pools of PA are often obscured by larger PA pools involved in intermediary metabolism (for example, in the endoplasmic reticulum). To reveal PA production at the cellular and subcellular levels, several biosensors featuring PA-binding domains (PABD) attached to fluorescent proteins have been reported [8]–[11]. Such probes relying on membrane translocation and a single fluorescence signal do not discriminate between real PA rises and changes in the thickness of the cell or membrane ruffling events, which would also affect fluorescence [12]. In addition, translocation sensors cannot be targeted, hampering the study of PA fluctuations in specific subcellular compartments.
In the present work, we have developed FRET sensors to monitor PA dynamics in the plasma membrane using the PA-binding domain (PABD) of the yeast protein Spo20 (residues 51–91) [9]. We found an inverse relation between plasma membrane PA levels and the FRET efficiency of the sensor. Interestingly, the studies carried out with the sensor indicated a redistribution of PA between the leading and trailing edges of migrating cells. In cells derived from oligodendrocytes and Schwann cells, PA levels were higher in the cell body than in the membrane processes involved in myelination. In contrast, DAG levels were lower in the cell body than in these membrane processes.

Inga kommentarer:
Skicka en kommentar